Semiquantitative mIBG Modified Curie Scoring Calculator
References:
- Yanik GA, Parisi MT, Shulkin BL, Naranjo A, Kreissman SG, London WB, Villablanca JG, Maris JM, Park JR, Cohn SL, McGrady P, Matthay KK. Semiquantitative mIBG scoring as a prognostic indicator in patients with stage 4 neuroblastoma: a report from the Children's oncology group. J Nucl Med. 2013 Apr;54(4):541-8. doi: 10.2967/jnumed.112.112334. Epub 2013 Feb 25. PMID: 23440556; PMCID: PMC5503147.
- Ady N, Zucker JM, Asselain B, Edeline V, Bonnin F, Michon J, Gongora R, Manil L. A new 123I-MIBG whole body scan scoring method--application to the prediction of the response of metastases to induction chemotherapy in stage IV neuroblastoma. Eur J Cancer. 1995;31A(2):256-61. doi: 10.1016/0959-8049(94)00509-4. PMID: 7718334.
Related Calculators:
More about the mIBG Semiquantitative Modified Curie Scoring Calculator
Overview
The mIBG Semiquantitative Modified Curie Scoring calculator is a specialized tool designed to assist in the assessment of metastatic disease burden in patients with neuroblastoma, a common extracranial solid tumor in children. The method is based on semiquantitative evaluation of 123I or 131I metaiodobenzylguanidine (mIBG) scintigraphy, which remains the gold standard for functional imaging in neuroblastoma staging, treatment response evaluation, and follow-up.
This calculator applies the principles of the Modified Curie scoring system, which divides the body into predefined anatomical regions and assigns a numeric score according to the extent of mIBG uptake in each region. By summing regional scores, clinicians obtain an overall measure of skeletal and soft tissue disease involvement, allowing for standardized reporting and longitudinal comparison.
The Modified Curie Scoring Method
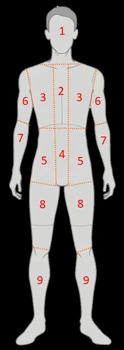
The original Curie scoring system was developed to quantify metastatic burden on mIBG scans by assigning each of 9 skeletal regions and soft tissue lesions a score from 0 to 3. The modified version retains the skeletal segmentation but adjusts scoring rules for greater reproducibility and applicability in cooperative group trials. The maximum possible score is typically 30 or 36, depending on whether both skeletal and soft tissue disease are included in the calculation.
- Score 0: No abnormal uptake
- Score 1: Uptake in one site within a region
- Score 2: Uptake in more than one site within a region but less than 50% of the region
- Score 3: Uptake in more than 50% of the region
The final Modified Curie score is the sum of all regional scores, providing a semiquantitative index of total disease burden.
Clinical Applications
The mIBG Semiquantitative Modified Curie Score is widely used in the management of neuroblastoma for:
- Baseline Staging: Quantifies initial metastatic burden for risk stratification.
- Response Assessment: Measures changes in disease extent after induction chemotherapy, surgery, or radiotherapy.
- Prognostic Evaluation: Higher post-treatment scores are often associated with increased relapse risk and poorer event-free survival.
- Clinical Trials: Serves as a standardized endpoint for response assessment in multicenter cooperative studies.
Advantages of the Modified Curie Scoring Calculator
- Standardization: Reduces inter-observer variability by using defined anatomical regions and scoring rules.
- Comparability: Facilitates direct comparison of patient scans over time and between institutions.
- Integration with Treatment Protocols: Aligns with many pediatric oncology cooperative group guidelines for neuroblastoma.
Limitations and Considerations
While the Modified Curie score is a valuable measure of metastatic disease burden, its accuracy depends on image quality, proper patient preparation, and consistent interpretation by experienced nuclear medicine physicians. The score reflects disease extent rather than metabolic activity, and it does not capture microscopic disease. Additionally, variations in mIBG uptake due to tumor biology or prior therapy may influence results.
Conclusion
The mIBG Semiquantitative Modified Curie Scoring calculator provides a structured, reproducible method for quantifying metastatic neuroblastoma on functional imaging. By transforming complex scintigraphic patterns into a single numeric value, it supports evidence-based clinical decisions, facilitates communication among multidisciplinary teams, and enhances comparability in research and clinical trial settings.